News & Press

David Pépin, PhD, Oviva Therapeutics, and Granata Bio featured in Time magazine series on The Age of Longevity
David Pépin, PhD, Oviva Therapeutics, and Granata Bio featured in Time magazine series on The Age of Longevity

Zeb Younes, Chief Regulatory Officer, CMC at Granata Bio, featured in the January 2026 issue of Regulatory Rapporteur
Granata Bio’s Chief Regulatory Officer, CMC, was featured in the January 2026 issue of Regulatory Rapporteur, exploring how confidence-based, risk-proportionate regulatory pathways can enable innovation across complex products—from brain-computer interfaces to digital CMC—without compromising patient safety.

ASRM Spotlight: AMH’s Expanding Therapeutic Potential Across Reproductive Medicine
Emerging research shows AMH is more than a marker of ovarian reserve, revealing an addressable therapeutic target with applications across menopause, oncofertility, and IVF stimulation.

Granata Bio Reports Strong Enrollment Momentum in Pivotal Phase III GRACE Study of Investigational Gonadotropin for Assisted Reproductive Technology
Granata Bio announced strong momentum in its pivotal Phase III clinical study, with multiple subjects already screened to evaluate the safety and efficacy of its investigational gonadotropin product for use in assisted reproductive technology (ART). The GRACE multicenter study is now actively enrolling across leading fertility centers throughout the United States.

ASRM Presentation Now Available: Anti-Müllerian Hormone (AMH) as a Therapeutic Target in Women’s Health
David Pepin, PhD, is reimagining the role AMH may play in future infertility treatment, aiming to give care teams expanded options in the clinic.

Is There an End in Sight to Reproductive Health's Drug Innovation Drought?
Reproductive health is a therapeutic area that has seen too little progress for too long.


Granata Bio Corporation and Georgetown Equity Partners Announce Joint Venture to Develop a Novel, Next-Generation Form of Follicle-Stimulating Hormone (FSH)
Granata Bio Corporation and Georgetown Equity Partners Announce Joint Venture to Develop a Novel, Next-Generation Form of Follicle-Stimulating Hormone (FSH)

CEO Evan Sussman Featured in Boston Globe Article: Medication madness, increasing involvement of private equity — why IVF costs so much, and how it may get worse
CEO Evan Sussman worked with The Boston Globe to highlight how a lack of innovation in reproductive health has negatively impacted affordability in IVF treatments and how Granata Bio and others are working to make infertility medications more accessible for those on the path to parenthood.



Women's Health Innovator Granata Bio Raises $15M Series A+ to Further Develop and Expand Reproductive Health Pipeline
Granata Bio Corporation announced a $15M Series A+ funding round. The round was exclusively supported by existing investors, including Gedeon Richter, CooperSurgical, Amboy Street Ventures, and other strategic investors. Granata has raised over $30 million to date.



IBSA Group and Granata Bio announces first patient screened in pivotal PROGRESS clinical trial of Progesterone-IBSA
IBSA Group and Granata Bio announces first patient screened in pivotal PROGRESS clinical trial of Progesterone-IBSA
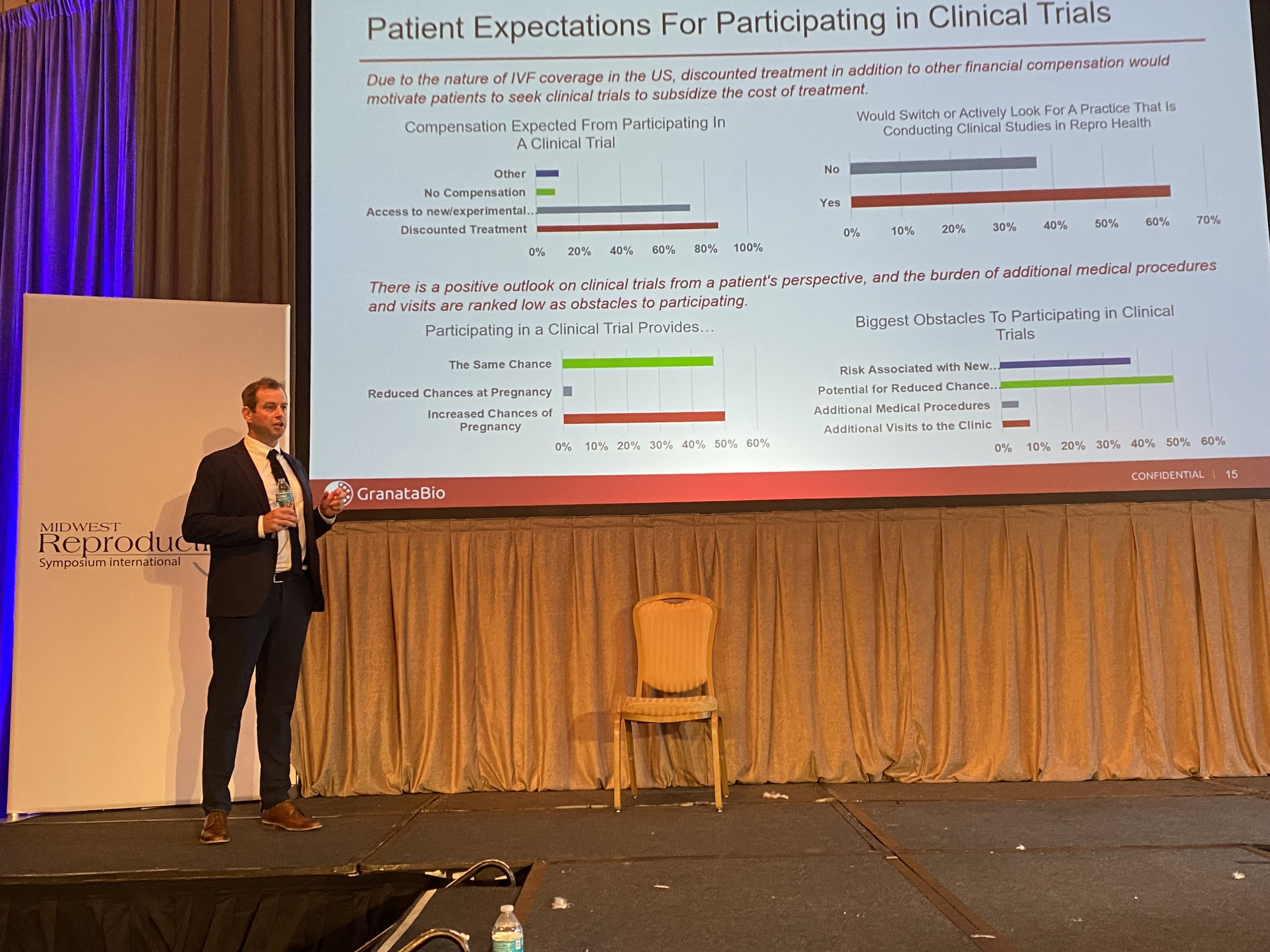
Granata Bio is Partnering with RESOLVE: The National Infertility Association
Granata Bio is partnering with RESOLVE: The National Infertility Association, to better understand clinical trials from the patients’ perspective

Granata Bio CEO, Evan Sussman to Moderate Panel on Innovation in Reproductive Health
Granata Bio is excited to have Dr. Denny Sakkas from Boston IVF, Dr. Lynn Westphal from Kindbody and Dr. Ken Carson from Tempus Labs, Inc. participate in a panel discussion on Innovation in Drug Discovery, R&D & Reproductive Health, moderated by our CEO, Evan Sussman. At this year’s Reproductive Health Innovative Summit on February 15-16 in Boston, MA.

